Ethanoic Acid and Sodium Hydroxide
Does ascorbic acid react with sodium. A When ethanoic acid reacts with sodium hydroxide a salt called sodium ethanoate along with water is formed.
What Is The Balanced Chemical Equation For The Neutralization Of Ch3cooh Acetic Acid And Naoh Sodium Hydroxide Quora
Titration of adding sodium hydroxide to ethanoic acid.
. Ethanoic acid and NaOH Sodium ethanoate and water are given as products by the reaction of ethanoic acid and aqueous NaOH. The balanced chemical reaction between ethanoic acid and sodium metal is shown below. Acetic acid weak reacts with.
CH3COOH OH- CH3COO- H2O Sodium. Write equation of he reaction involved b What happens when vegetable oils are hydroenate. Organic acids also react with strong alkali metals to form strong basic salts with the liberation of hydrogen gas.
Slowly add a 1M solution of. 25 mL of a 0095M of acetic acid was diluted to 100mL with deionized water and titrated with 0101M of NaOH. Nanoleaf canvas expansion pack.
The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid. Sodium ethanoate is a weak basic saltethanoic acid and So. Ethanoic acid contains carboxylic acid as a functional group.
The equation for the reaction occurring is CH3COOH aq NaOH aq -. Answer 1 of 5. The chemical equation of the reaction is as follows.
Ethanoic acid and just about any other acid will react with sodium hydroxide rather than dissolve in it. Suggest the pH at the equivalence point either as. Ad Expertise on Every Level to Craft Science Technology Solutions in Life Science.
This reaction is considered a. A What happens when ethanoic acid reacts with sodium hydroxide. Ethanoic acid is a weak organic acid that combines with sodium metal to generate sodium ethanoate CH 3 COO-Na also known.
Aqueous ethanoic acid shows a pH value below than 7 usually 3-7 but may change due to concentration. For the titration of ethanoic acid CH3COOH with sodium hydroxide NaOH a. How do you neutralize acetic acid and sodium hydroxide.
C H 3 C O O H N. Sodium hydroxide is a strong electrolyte so. All chemical reactions in which ethanoic acid participates occur due to this active carboxylic acid group.
Reaction of ethanoic acid CH 3 COOH with sodium Na. Sodium hydroxide NaOH a strong base will react with lactic acid C3H6O3 a weak acid to form water and aqueous sodium lactate NaC3H5O3. CH3COOH NaOH CH3COONa H2O Acetic acid is a weak electrolyte.
A The net ionic equation for the aqueous neutralization reaction between acetic acid and sodium hydroxide is different from that for the reaction between hydrochloric acid and. Strong bases are considered strong. Ad Expertise on Every Level to Craft Science Technology Solutions in Life Science.
The reaction of Sodium hydroxide and Acetic acid also called Ethanoic acid represents a net ionic equation involving a strong base and a weak acid. The reaction of ethanoic acid with sodium metal results in the formation of. Acetic acid reacts with aqueous sodium hydroxide and give sodium ethanoate and water as products.
Market Leader in Innovative Organic Inorganic Chemicals. Ethanoic acid is commonly known as acetic acid. Identify solution should be used as a titrant.
Iot temperature and humidity sensor. Sodium ethanoate is a salt and soluble in water. Market Leader in Innovative Organic Inorganic Chemicals.
2005 yamaha bruin 350 oil capacity. The reaction of --α-pinene 1 with sodium nitrite-acetic acid gave principally 24-2-nitromentha-168-diene 2 and smaller amounts of 24. Slowly add acetic acid to a container of cold water to form a 110 dilution of acid to water.

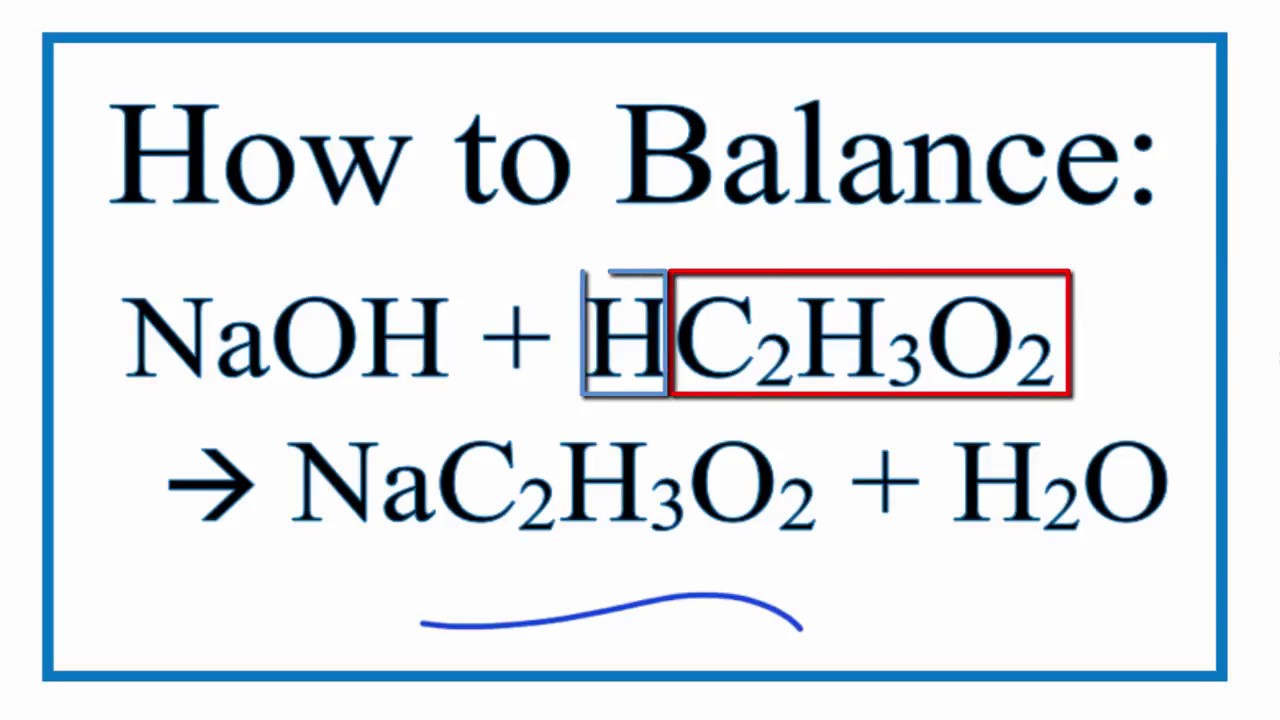
How To Balance Naoh Hc2h3o2 Nac2h3o2 H2o Sodium Hydroxide Plus Acetic Acid Youtube

Net Ionic Equation For Naoh Ch3cooh Strong Base And Weak Acid
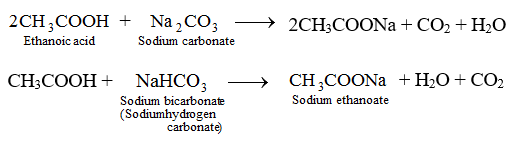
Physical Amd Chemical Properties Of Ethanoic Aacid Chemistry Knowledgeuniverseonline Com

No comments for "Ethanoic Acid and Sodium Hydroxide"
Post a Comment